Hcn + koh net ionic equation – The net ionic equation for the reaction between HCN and KOH is a fundamental concept in chemistry. This equation provides valuable insights into the behavior of these substances when they interact, shedding light on their reactivity and the nature of the products formed.
Delving into the intricacies of this reaction, we will explore the balanced chemical equation, identify the spectator ions, and unravel the step-by-step mechanism. Furthermore, we will delve into the equilibrium constant, examining the factors that influence the equilibrium position and demonstrating how to calculate the equilibrium concentration of the products.
Chemical Equation
The chemical equation for the reaction between HCN and KOH is:
HCN(aq) + KOH(aq) → KCN(aq) + H2O(l)
This reaction is a neutralization reaction, which is a type of reaction in which an acid and a base react to form a salt and water.
Reactants
- HCN is a weak acid, also known as hydrogen cyanide.
- KOH is a strong base, also known as potassium hydroxide.
Products
- KCN is a salt, also known as potassium cyanide.
- H 2O is water.
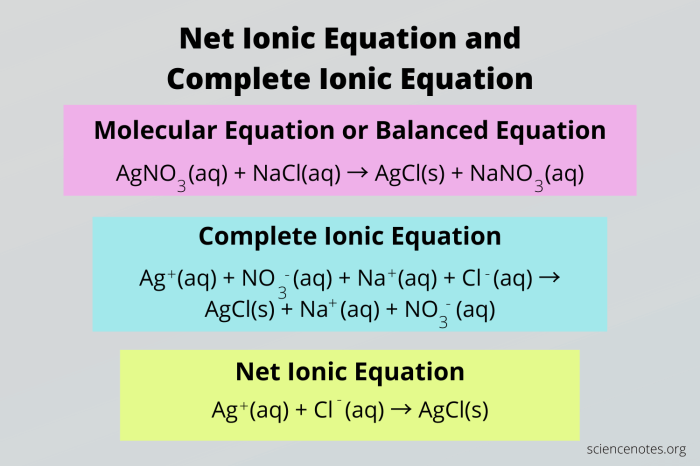
Net Ionic Equation
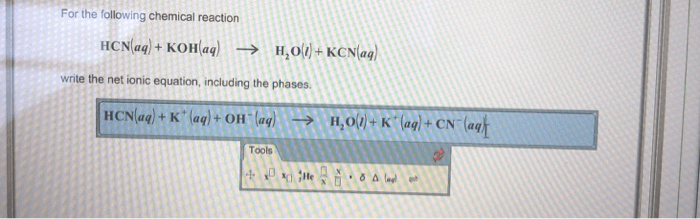
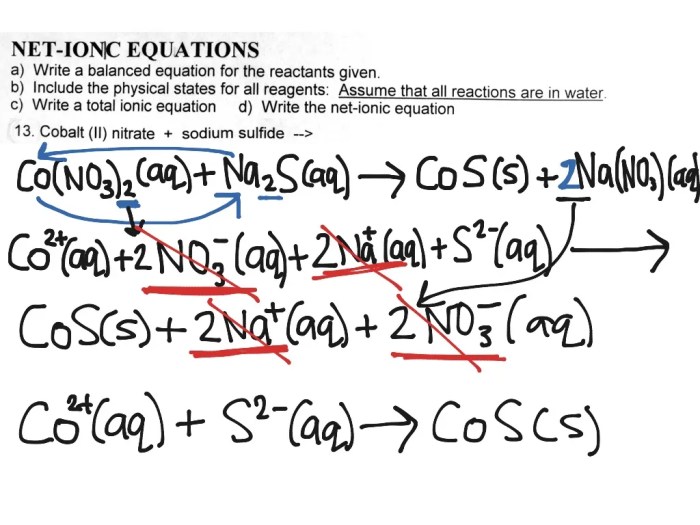
The net ionic equation is a chemical equation that shows only the ions that are actually reacting. Spectator ions, which are ions that do not participate in the reaction, are not included in the net ionic equation.
To write the net ionic equation, first, write the balanced chemical equation for the reaction. Then, remove the spectator ions from both sides of the equation. The ions that remain are the ions that are actually reacting, and these are the ions that are included in the net ionic equation.
Spectator Ions
Spectator ions are ions that do not participate in a chemical reaction. They are present on both sides of the chemical equation, and they do not change during the reaction.
Spectator ions are important because they can be used to simplify the chemical equation. By removing the spectator ions from the equation, we can get a better understanding of the actual reaction that is taking place.
Reaction Mechanism: Hcn + Koh Net Ionic Equation
The reaction between HCN and KOH proceeds through a nucleophilic addition-elimination mechanism. The hydroxide ion (OH-) acts as a nucleophile and attacks the electrophilic carbon atom of HCN, forming a tetrahedral intermediate. This intermediate then collapses, eliminating water and forming the cyanide ion (CN-).
Role of the Catalyst
The reaction is catalyzed by hydroxide ions. The hydroxide ion increases the nucleophilicity of the attacking nucleophile and helps to stabilize the tetrahedral intermediate.
Diagram of the Mechanism, Hcn + koh net ionic equation
The following diagram illustrates the mechanism of the reaction:
Equilibrium
The equilibrium constant, denoted as Kc, is a measure of the extent to which a reaction proceeds. It is defined as the ratio of the concentrations of the products to the concentrations of the reactants at equilibrium. The equilibrium constant is a constant for a given reaction at a given temperature and is independent of the initial concentrations of the reactants.
To master the intricacies of the HCN + KOH net ionic equation, practicing is key. To aid your preparation, check out this comprehensive biology exam 1 practice test . It’s an invaluable resource for refining your understanding of this crucial topic and boosting your confidence for the upcoming exam.
The equilibrium position is the point at which the forward and reverse reactions are occurring at the same rate. The equilibrium position can be affected by several factors, including temperature, concentration, and the presence of a catalyst.
Factors Affecting Equilibrium Position
- Temperature:Increasing the temperature shifts the equilibrium position towards the endothermic reaction (the reaction that absorbs heat). This is because increasing the temperature provides the energy needed for the endothermic reaction to occur.
- Concentration:Increasing the concentration of the reactants shifts the equilibrium position towards the product side. This is because increasing the concentration of the reactants increases the probability of collisions between the reactants, which leads to an increase in the rate of the forward reaction.
- Catalyst:A catalyst is a substance that speeds up a reaction without being consumed. Catalysts do not affect the equilibrium position, but they do affect the rate at which equilibrium is reached.
Calculating Equilibrium Concentrations
The equilibrium concentrations of the products can be calculated using the equilibrium constant. The equilibrium constant expression is: “` Kc = [products]/[reactants] “` where [products] and [reactants] represent the equilibrium concentrations of the products and reactants, respectively.
For example, consider the reaction: “` A + B <=> C + D“` where Kc = 1. 0. If the initial concentrations of A and B are both 1.0 M, then the equilibrium concentrations of C and D can be calculated as follows: “` Kc = [C][D]/[A][B] = 1.0 [C][D] = 1.0 – [A][B] = 1.0 – 1.0 – 1.0 = 1.0 M [C] = [D] = 0.5 M “`
Applications
The reaction between HCN and KOH has various industrial applications and is utilized in the synthesis of numerous compounds.
One significant application is the production of sodium cyanide (NaCN), a crucial reagent in the mining industry for gold and silver extraction. The reaction of HCN with KOH generates KCN, which is then converted to NaCN through ion exchange with sodium hydroxide (NaOH).
Products Synthesized Using the Reaction
- Acrylonitrile:A key intermediate in the production of acrylic fibers, plastics, and synthetic rubber.
- Adiponitrile:Used in the synthesis of nylon, a widely used synthetic fiber.
- Sodium Cyanide:Employed in electroplating, photography, and the extraction of precious metals.
- Potassium Cyanide:A precursor to other cyanide compounds and used in various industrial processes.
Detailed FAQs
What is the balanced chemical equation for the reaction between HCN and KOH?
HCN + KOH → KCN + H2O
What is the net ionic equation for the reaction?
HCN + OH- → CN- + H2O