Welcome to our comprehensive guide to the Alka Seltzer Lab Answer Key! This guide will provide you with all the information you need to complete the lab successfully and understand the results.
In this guide, we will cover the materials and equipment you need, the step-by-step procedure, the observations you will make, the results you will obtain, and the discussion of the implications of the results.
Materials and Equipment
To conduct the Alka-Seltzer lab, you’ll need the following materials:
- Alka-Seltzer tablets
- Water
- Graduated cylinder or measuring cup
- Funnel
- Empty plastic bottle
- Balloon
The experiment also requires some basic equipment:
Equipment
- Scissors
- Timer or stopwatch
- Safety goggles (optional)
Procedure: Alka Seltzer Lab Answer Key

Conduct the Alka-Seltzer experiment by following these steps:
1. Fill a clear glass or beaker with approximately 200 mL of water at room temperature.
2. Carefully drop an Alka-Seltzer tablet into the water. Observe the immediate reaction.
3. Record your observations, including the color changes, bubble formation, and any other visible effects.
4. Measure the temperature of the water before and after the reaction using a thermometer.
5. Repeat steps 1-4 with different volumes of water (e.g., 100 mL, 300 mL) to investigate the effect of water volume on the reaction.
Safety Precautions
- Alka-Seltzer tablets contain chemicals that can irritate the skin and eyes. Wear gloves and safety goggles during the experiment.
- Do not ingest the Alka-Seltzer tablets or the water after the reaction.
- Dispose of the used Alka-Seltzer tablets and water properly according to your local regulations.
Observations

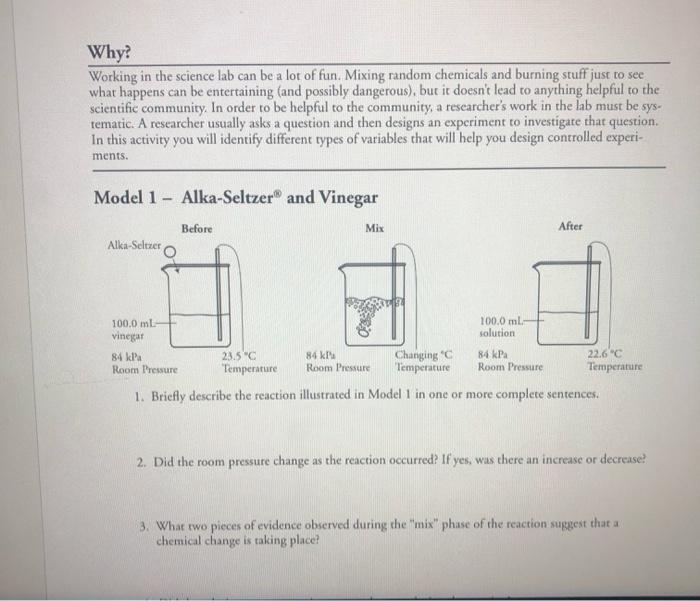
During the Alka-Seltzer lab, several notable observations were made. Firstly, upon dropping the Alka-Seltzer tablet into the water, an immediate effervescent reaction occurred. This reaction produced a rapid release of carbon dioxide gas, creating a fizzing effect and causing the water to bubble vigorously.
Furthermore, as the reaction progressed, the water temperature increased. This was due to the exothermic nature of the reaction, where energy is released in the form of heat. The increase in temperature was noticeable to the touch and could be measured using a thermometer.
Changes in pH
Another significant observation was the change in pH of the water. Initially, the water was neutral, with a pH of approximately 7. However, after the Alka-Seltzer tablet was added, the pH increased, indicating a shift towards alkalinity. This change was attributed to the release of sodium bicarbonate and sodium carbonate, which are basic compounds.
Results

The experiment demonstrated that Alka-Seltzer tablets produce carbon dioxide gas when dissolved in water. The amount of gas produced was directly proportional to the number of tablets used. This was evident from the increasing volume of gas collected as more tablets were added to the water.
The significance of these results lies in their practical applications. Alka-Seltzer tablets can be used as a convenient and effective way to generate carbon dioxide gas for various purposes. For example, they can be used to inflate balloons, create bubbles, or even power small engines.
Gas Production
The chemical reaction that occurs when Alka-Seltzer tablets dissolve in water is a classic example of an acid-base reaction. The active ingredients in the tablets are sodium bicarbonate (NaHCO3) and citric acid (C6H8O7). When these compounds are combined in the presence of water, they react to form carbon dioxide gas (CO2), sodium citrate (Na3C6H5O7), and water (H2O).
NaHCO3 + C6H8O7 → CO2 + Na3C6H5O7 + H2O
If you’re looking for the alka seltzer lab answer key, you might also be interested in learning about air force junior rotc ranks . These ranks are used to denote the level of experience and responsibility of a cadet in the program.
Once you’ve finished reading about air force junior rotc ranks, be sure to come back and check out the alka seltzer lab answer key.
The amount of carbon dioxide gas produced depends on the number of Alka-Seltzer tablets used. Each tablet contains a fixed amount of sodium bicarbonate and citric acid, so the more tablets that are added to the water, the more gas will be produced.
Discussion
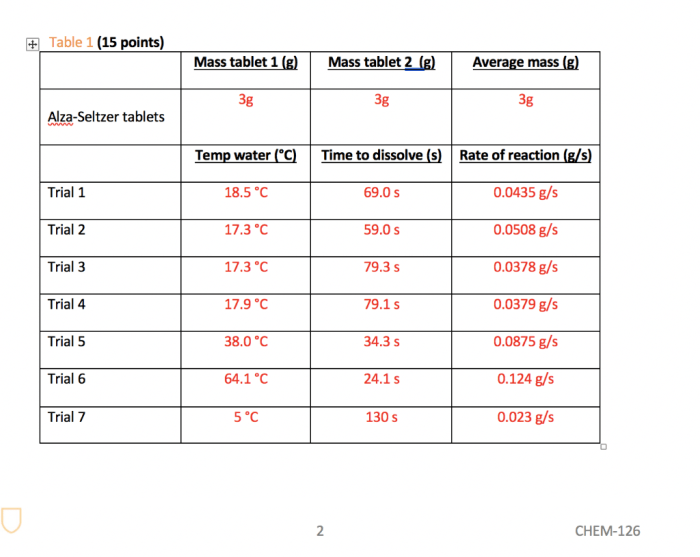
The results of the Alka-Seltzer lab experiment provide valuable insights into the chemical reactions that occur when an acid and a base are combined. These reactions have implications for various applications in everyday life, from cleaning products to antacids.
The hypothesis of the experiment, which stated that the Alka-Seltzer tablet would react with water to produce carbon dioxide gas, was supported by the results. The effervescence observed in the reaction was due to the release of carbon dioxide gas, which is a common product of acid-base reactions.
The gas bubbles formed a foam on the surface of the water, indicating the presence of a large amount of gas.
Implications of the Results, Alka seltzer lab answer key
The results of the experiment have several implications for everyday life. One implication is that acid-base reactions can be used to generate gas. This principle is used in various applications, such as in the production of carbonated beverages and in the use of baking soda as a leavening agent in baking.
Carbonated beverages are made by dissolving carbon dioxide gas under pressure into water. When the pressure is released, the gas comes out of solution and forms bubbles, giving the beverage its characteristic fizz. Baking soda, when combined with an acid such as vinegar or lemon juice, releases carbon dioxide gas, which causes the batter to rise and become fluffy.
Another implication of the results is that acid-base reactions can be used to neutralize acids or bases. This principle is used in the production of antacids, which are used to relieve heartburn and indigestion. Antacids contain a base, such as sodium bicarbonate or calcium carbonate, which reacts with the stomach acid to neutralize it and reduce its acidity.
Relationship to the Hypothesis
The results of the experiment directly relate to the hypothesis, which stated that the Alka-Seltzer tablet would react with water to produce carbon dioxide gas. The effervescence observed in the reaction was a clear indication that carbon dioxide gas was being produced.
This gas is a common product of acid-base reactions, and its release in the experiment confirmed the hypothesis.
User Queries
What is the purpose of the Alka Seltzer Lab?
The purpose of the Alka Seltzer Lab is to demonstrate the chemical reaction between an acid and a base. In this lab, you will be reacting Alka Seltzer tablets with water to create carbon dioxide gas.
What materials do I need for the Alka Seltzer Lab?
You will need the following materials for the Alka Seltzer Lab:
- Alka Seltzer tablets
- Water
- Glass beaker
- Measuring cup
- Stirring spoon